Transfection Reagents for Cell and Gene Therapy
Gene therapy involves the transfer of genetically modified materials into a patient's cells to correct abnormal genes associated with diseases (Sun, 2017). Recent advancements in gene therapy have expanded its applicability to a wide range of diseases as the number of potential gene targets continues to grow. However, one of the critical challenges in developing effective gene therapies is the mechanism of delivery, which requires efficient transfer systems to mediate high gene expression (Durymanov, 2018).
Viral vectors, particularly adeno-associated virus (AAV) and lentivirus, have emerged as safe and effective delivery mechanisms for gene therapy applications (Naso, 2017). AAVs are popular due to their favorable therapeutic profile, mild toxicity, low immunogenicity, and as they are already approved for use in numerous clinical trials (Daya, 2008). Lentiviral vectors offer the advantage of accommodating larger nucleic acids and integrating into the host genome for long-term expression, providing highly targeted approaches with minimal safety concerns (Milone, 2018). However, the production of viral vectors relies heavily on transfection, making the selection of the right transfection reagent crucial for achieving high-efficiency cell lines with low cytotoxicity for both clinical and research applications.
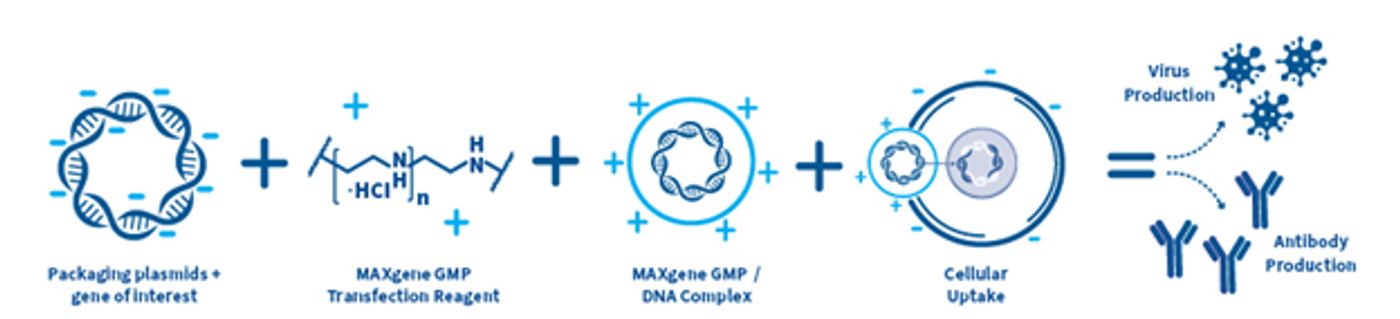
Polyethylenimine (PEI) has a well-established history as a gold standard transfection reagent for both in vivo and in vitro gene transfer (Zakeri, 2018). PEI, a polycationic polymer, forms positively charged complexes with nucleic acids, facilitating their interaction with cell surfaces, endocytosis, and transport into the cytoplasm, ultimately leading to nuclear delivery. PEI exhibits excellent tolerance with low cytotoxicity in various cell types, including both adherent and suspension cell culture systems (Sawant, 2012).
Kyfora Bio, the bioprocessing spin-off of Polysciences, is backed by 60+ years of high-quality specialty chemical development, manufacturing, and application expertise, and serving as a trusted partner for pharmaceutical and biotech companies engaged in gene and cell therapy product development. Over the past few years, Kyfora Bio has developed a line of research-grade (PEI MAX™ and Transporter 5™) and GMP-grade (MAXgene™ GMP) PEI transfection reagents suitable for seamless transitions from process development to clinical trials and commercial manufacturing of viral vectors. These products have been widely cited for their cost-effective suitability in viral vector manufacturing (Bhat, 2018; Grieger, 2016).
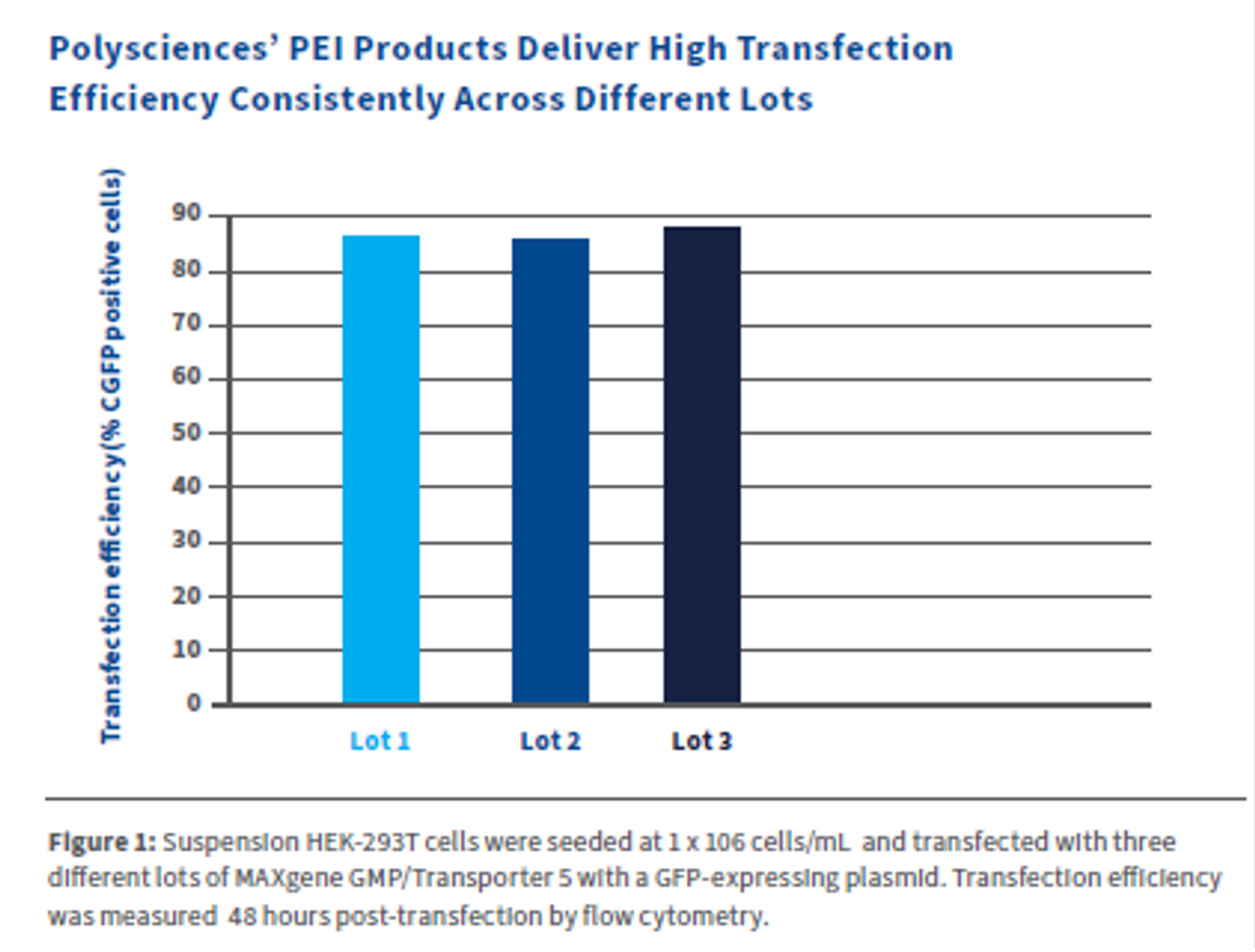
Kyfora Bio’s PEIs are characterized by a high density of protonatable amino groups, with every third atom being amino nitrogen, which imparts a robust buffering capacity at nearly any pH. Consequently, once inside endosomes, PEI disrupts vacuoles, releasing genetic material into the cytoplasm. This endosomal escape capability, combined with the ability to form stable complexes with nucleic acids, makes Polysciences' PEI a highly efficient gene delivery vector with minimal cytotoxicity. These products maintain consistent transfection efficiency from one lot to another, resulting in high titer yields in both small- and large-scale cultures.
Biotherapeutics represent an effective treatment option for a diverse range of diseases, and the development process plays a pivotal role in ensuring the quality and consistency of the final product. As a trusted manufacturing partner for cell and gene therapy manufacturers for over a decade, Polysciences recognizes the importance of high-quality raw materials throughout the development journey, from early stages through pivotal trials to the safe and effective delivery of drugs to patients.
Daya, S., Kenneth, B. (2008). Gene Therapy using adeno-associated virus vectors. Clin Microbiol Rev. 21 (4). 583-93.
Durymanov, M., Oshua, R. (2018). Non-viral delivery of nucleic Acids: Insight In to mechanisms of overcoming intracellular barriers. Front Pharmacol 9 (971).
Grieger, J., Stephen, S., Richard, S. (2016). Production of recombinant adeno-associated virus vectors using suspenstion HEK293 cells and continuous harvest of vector from the culture media for GMP FIX and FLT1 clinical vector. Mol Ther 24 (2) 287-297.
Milone, M., Una, O. D. (2018) Clinical use of lentiviral vectors. Leukemia 32: 1529-154.
Naso, M., Brian, T., William, P., William, S. (2017) Adeno-associated virus (AVV) as a vector for gene therapy. BioDrugs 31 (4), 317-334.
Sun, W., Wei, Z., Anton, S. (2017). Drug discovery and development for rare genetic disorders. Am J Med Genet A. 173 (9), 2307-2322.
Abbas, Z., Mohammad, A. J. K., Nasrin, B., Vahid, B., Seyyed, M. M., Seyyed ,A. R. H., Ayoob, K. Z., Ali, M. A., Amir, S., Esmail M., Sara J., Ahmad M. (2018).Polyethylenimine-based nanocarriers in co-delivery of drug and gene: a developing horizon.” Nano Rev Exper. 9 (1).