Opioid Addiction Can be Controlled Using an In-Brain Chip Technology: First U.S. Clinical Trial
Opioid addiction is a chronic disease that can cause significant health, social, and economic problems. Opioids are a class of drugs that act in the nervous system to produce feelings of pleasure and pain relief. The overdose of opioids negatively affects a person's personal and professional relationships. Some opioids are legally prescribed by healthcare providers to manage severe and chronic pain.
According to the US Department of Health & Human Services, there were 47,600 of opioids overdose deaths, and more than130 people died from opioid-related drug overdoses in 2016 and 2017.
Researchers in China conducted the first clinical trial of DBS for methamphetamine addiction at Shanghai's Ruijin Hospital in China, along with tests for opioid addicts.
The patients are resistant to the various treatments for opioid addiction. Researchers at the West Virginia University Rockefeller Neuroscience Institute (RNI) and West Virginia University Medicine are conducting the first clinical trial in the US that uses deep brain stimulation (DBS) to treat opioid addiction.
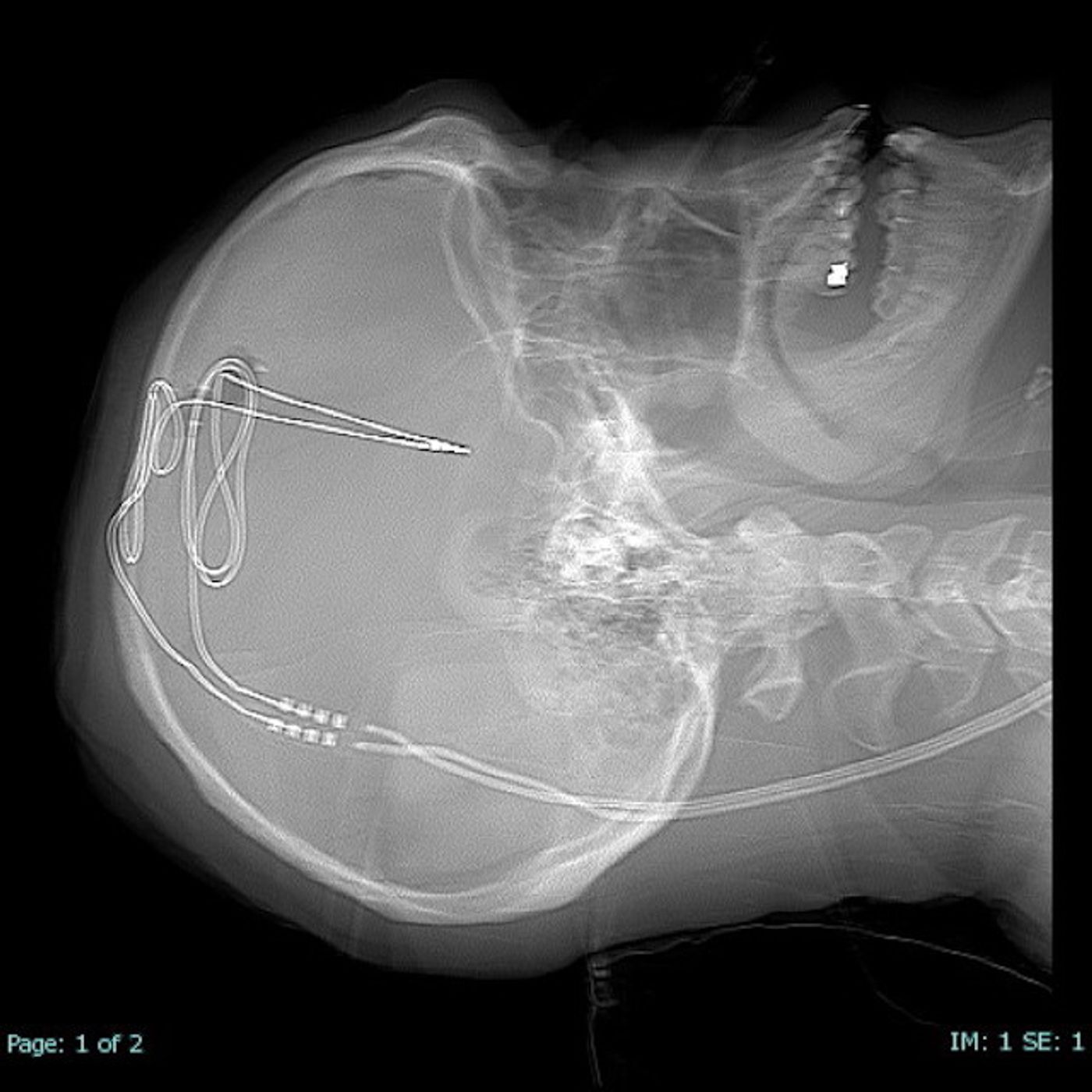
Media-kit-wvu-Rockefeller-neuroscience-institute: Implantation of tiny electrodes into specific brain areas.
“Despite our best efforts using current, evidence-based treatment modalities, there exist several patients who don’t respond. Some of these patients remain at very high risk for ongoing catastrophic health problems and even death. DBS could prove to be a valuable tool in our fight to keep people alive and well,” said Dr. James Berry, interim chair of the WVU Department of Behavioral Medicine and Psychiatry and director of Addiction Services at RNI.
What is DBS?
DBS, or brain pacemaker surgery, place tiny electrodes into specific brain areas that are involved in creating addictions and self-control behaviors. These electrodes will send electric impulses at frequencies carefully tuned to enhance positive brain waves and disrupt harmful signals.
This in-brain chip provides information about opioid cravings in patients; it will also be manipulated by scientists to arrest these cravings by sending counter-impulses to this region. The inputs will help scientists understand more about the addiction mechanism in the patient's brain.
RNI's first trial included four participants. The patients who have undergone thorough courses of treatment across several programs and yet continue to suffer from addiction participated in the study. Another similar trial of DBS in 60 patients with refractory opioid dependence is underway in China, as reported in the BMJ Open in 2019.
The U.S. Food and Drug Administration has approved DBS for treating patients with Parkinson's disease, major depressive disorder, and epilepsy, among others, and has shown promising results.