Bonding Molecules with UV Light
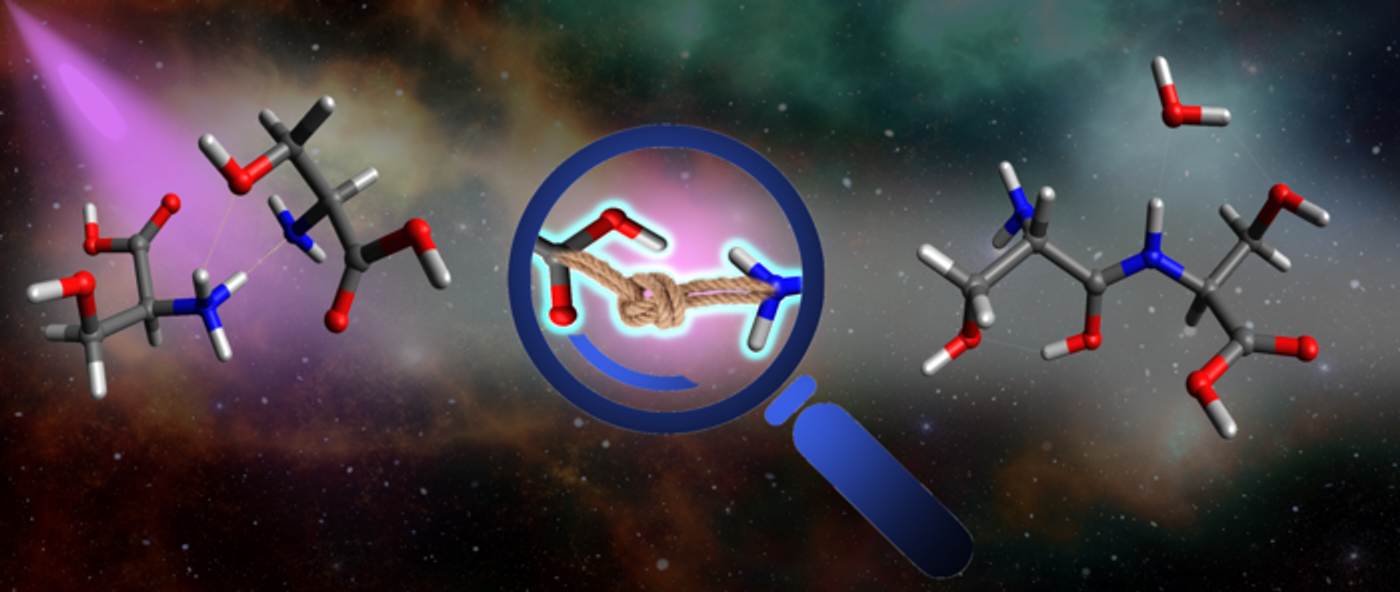
Illustration of intracluster bond formation, cluster containing two biomolecules, being exposed to UV light (left), causes a chemical bond forming between the two molecules (center), resulting in a single, large, and more complex biomolecule (right). (Credit: Dario Barreiro Lage, Madrid University)
Searching for life beyond Earth starts at the complex molecular level, and an international team of researchers led by Bar-Ilan University in Israel examine how to form chemical bonds between molecules within molecular clusters using ultraviolet (UV) light. This study holds the potential to help scientists better understand how complex life forms are in the first place, both on Earth and possibly beyond it.
For the study, the researchers examined a process known as intra-cluster bond formation (ICBF), which involves joining molecules through chemical bonds before the cluster disappears as molecular clusters have been shown to do due to their weak forces trying to keep them together.
The amino acid cluster of serine is the most widely examined cluster due to their ability to form clusters that possess eight molecules, which has led scientists to hypothesize about life’s origins possibly owing its very existence to serine. However, scientists have yet to successfully observe the formation of bonds within these clusters.
In a previous study led by this same group of researchers, the team observed cluster formation of peptide bonds that possessed four serine dipeptides that became hot when they collided but observed no such reaction involving serine clusters. They study concluded that if dipeptide could form from two serine molecules, then polymers—the substances that make comprise proteins, nucleic acids, and cellulose, also known as the building blocks of life—could happen more easily. It is this most recent study that such a method might have been produced.
“The dream of photochemistry is to use light to drive chemical reactions that are not likely to occur otherwise. The present experiments show a beautiful example of such a process,” said Dr. Yoni Toker, who is a senior lecturer in the Department of Physics at Bar-Ilan University, and a co-author on the study.
Using the French DESIRS beamline in SOLEIL synchrotron, the researchers were able to conduct their experiment, which allowed them to both create and choose specific clusters, then irradiate them with synchrotron light, followed by measuring the resulting fragments. On top of that, more complicated experiments can be conducted where research can mass-select fragments and then excited via collisions.
“We were hoping to see some evidence of bond formation in large serine clusters, such as the magic octamer,” said Ori Licht, who is a member of the Toker Lab, and lead author of the study. “But we were surprised to see bond formation occurring in clusters containing just two serine molecules after the absorption of a UV photon. We were even able to use MS3 measurements to confirm that the fragments we observed are indeed products of intra-cluster bond formation.”
The team discovered that peptide bond formation had happened upon measuring the difference between the serine dimer and the serine dipeptide, whose measurements were then confirmed using state-of-the-art quantum chemical calculations, which were conducted by the research group of Sergio Diaz Tendero of Madrid University. The team calculated the cluster’s excited state dynamics, discovering that peptide bond formation is the resulting evolution of the electronic states.
“In other words, our work provides both the experimental and theoretical framework for how one of the most important biophysical reactions can be triggered abiotically inside molecular clusters by the absorption of ultra-violet light," said Dr. Toker.
Lab-synthesized molecules produce equal amounts of left-handed versus right-handed molecules, whereas all amino acids, the building blocks of life, are left-handed. Going forward, the researchers wish to find out if the observed intro-cluster bond formation process prefers either left- or right-handed molecules.
Sources: Angewandte Chemie, Britannica, EurekAlert!
As always, keep doing science & keep looking up!